Asia-Pacific Forum on Science Learning and Teaching, Volume 15, Issue 2, Article 11 (Dec., 2014) |
Data analysis and interpretation
Physical environment of the school
The building of the school under study has six rooms along with a small kitchen located separately. Initially this kitchen was used for preparing mid-day meal for children under the ‘Mid-day Meal Scheme’ a government sponsored / initiated popular scheme. It involves provision of lunch on working days in schools. The main objective of the programme is protecting children from classroom hunger by providing a hot cooked balanced meal.
Figure 2. School building
The children of classes VI, VII and VIII are allotted separate classrooms. There is a Science and Mathematics activity room, science and mathematics kits have been provided under Sarva Shiksha Abhiyan(SSA). SSA is a scheme launched by Government of India to provide useful and relevant elementary education for all children in the 6-14 age groups. These kits contain consumable and non-consumable items, whenever required funds are being provided to get the consumable items. It may be mentioned that there are still many schools in India which do not have separate classrooms, and where children of different grades are made to sit in separate corners of a single large hall.
There is no separate library but 300 books are available which are kept in a cupboard. These books include dictionary of different languages, story books and novels by Indian authors.
There is a bore- well from which water is supplied for gardening and other purposes. This water is used by staff and students for drinking purposes. The rooms have fans and lights (bulbs) but most of the time when there is no electricity, the rooms get darker and the visibility becomes low.
Figure 3. Low visibility in classroom
There are five teachers including school in-charge and only one non-academic staff who looks after clerical and other secretarial work. There are 21, 19 and 18 children in classes VI, VII and VIII respectively, the total number of children is 58. Same –gender seating is preferred i.e boys prefer to sit with boys and girls prefer to sit with girls only, boys and girls hesitate in interacting freely with each other.
Classroom activities and observations
Apart from doing non-academic supervisory work, the in-charge of school was teaching science and mathematics to classes VI, VII and VIII. This is what researcher has observed:
“The teacher announces the topic from science textbook. She asks a student to read a paragraph from the textbook and she explains its meaning. Then she asks another student to read the next paragraph and explains the meaning and this goes on. Rest of the students do silent reading. The teacher writes questions and answers on the blackboard and students copy them. Sometimes teacher recapitulate the answers. Some students answer the questions orally and sometimes they copy answers from textbook”.
Researcher to the teacher: I would like to know why children are simply reading the activities. Why they are not allowed to perform activities? You are not even demonstrating any activity.
Teacher: I have to complete the syllabus in time. Activities take lots of time. So activity based teaching is not possible.
It was basically a teacher-centred class and most of the time one-way transaction was going on. In this school, teacher- pupil ratio was 1:20, very ideal. Such a ratio is rare in a country like India. No inquiry based learning was going on. However, it may be mentioned that inquiry based learning is a pedagogical method, developed during the discovery learning movement of the 1960s as a response to traditional forms of instruction - where people were required to memorise information from instructional materials (Bruner,1961).
Classroom experiences: Academic memories
It would be worthwhile to make a mention of some real classroom experiences in the form of examples, where inquiry based learning covers a wide range of approaches to teaching-learning. On the first day her visit in the class, children were a little hesitant in interacting with her and hence it took them some time to understand each other. To encourage frank conversation, the researcher remarked with a smile: ‘We are friends; we will try to explore and learn science together.’
The researcher’s focus was on science process skills. These are the skills that facilitate active students participation, students develop the sense of understanding responsibility in their own learning, acquire research ways and discovery skills, students ensure thinking and behave like a scientist. It is noteworthy that in science, process skills are the building – blocks of critical thinking and inquiring (Ostlund, 1992).
Example1 - Prior knowledge about the concept
In class VIII, researcher’s plan was to discuss a chapter on ‘Metals and Non-Metals’ (Textbook of Science, Class VIII, 2008). While inquiring children expressed they have already completed this chapter. To know whether they have really understood this chapter, she wrote the following basic multiple choice question on the blackboard and asked children to answer.
Which one of the following is not a metal?
a) Copper
b) Sulphur
c) Aluminium
d) Iron
The researcher got various responses, but none answered ‘sulphur’. She asked few more questions related to this concept orally which they could not answer. This helped her in assessing prior knowledge of learners (Details given in example VII).
Since, the researcher had gone to class with necessary preparation; she had carried some materials such as iron nail, a wire of copper, aluminium foil, zinc granules, silver ring, gold (bangle, which she was wearing), sulphur, coal etc. She showed these materials to students and asked them to identify metals and non-metals on the basis of their physical properties, which they had already studied in class VI.
Some students could identify metals but not ‘zinc’ which they all had seen for the first time. She encouraged the students who had better understanding of the concept to explain it to other students.
Researcher asked the students, “What makes you call these things metals?”
Students – They are hard, they shine, and they make a ting-ting sound when we hit them.
Researcher demonstrated the activities by involving students and showed them how metals are different from non-metals on the basis of their physical properties by following a learner-centred classroom approach. Researcher valued students’ experiences and their active participation. Next day, she discussed about the chemical properties of metals. One example which she is highlighting here is the nature of metallic oxide. She wanted to burn a piece of magnesium ribbon. Since she was not carrying any heating device, she told all the children to assemble in the kitchen [earlier used for cooking mid-day meal]. With the help of gas stove, they could burn the magnesium ribbon in seconds and collected the ash.
Children were excited to see burning of magnesium ribbon which they had never experienced before.
Since researcher had told them not to stare at burning magnesium ribbon because its light is dangerous for eyes, they were whispering to each other – “do not look at burning magnesium ribbon.”
Researcher asked them, “Can you name the ash formed”?
(She asked this question deliberately, because in the beginning they had said that they know about this concept.)
They all gave her blank looks.
Researcher told one of the students to put a few drops of water in this ash.
Her next question was, “How can we test the nature of this solution?”
They were looking at her and also at each other with blank faces.
Students – We don’t know. In between she could hear Kanchan (name of the student) saying with hesitation, “with litmus paper.” “Good”, she said .You have studied about indicators in your Class VII
Students – We don’t remember.
Researcher – Did anyone demonstrate or allow you to perform activities in Class VII?
Students–No!
She provided blue and red litmus paper to students and told all of them to try the nature of the solution with blue and red litmus paper.
Kanchan took blue litmus paper and dipped it in the solution.
Students – There is no change in the colour of blue litmus paper.
Jyoti – Let us try with red litmus paper. [She dips red litmus paper in the solution]. Wow! It is turning blue.
Researcher – Why has red litmus paper turned blue?
Students – No answer.
She told them to recall your Class VII chapter “Acids, bases, and salts.”
Since syllabus at upper primary stage (classes VI, VII and VIII) is developed in a spiral form and researcher could realise that children are not aware about the previous concepts, decided to discuss the chapter of class VII “Acid, Bases and Salts” with them. Now it was clear to her that they do not have any prior knowledge to understand this concept i.e nature of metallic oxide. For this the researcher followed activity based approach. She demonstrated and also allowed children to perform all the activities given in this chapter; it took her almost a week to discuss these concepts. When she felt they are comfortable with all the concepts given, she proceeded further.
Researcher-Can you now tell me the nature of metallic oxide?
Students-We now know the solution is basic in nature because red litmus paper turned blue and there was no change in the colour of the blue litmus paper.
Researcher - Can you think of any other test with which we can say that the solution is basic in nature?
Anil – He went outside the classroom and collected China rose flowers from the garden. He did not pluck the flower rather collected withered flowers from ground.
(This showed children has concern towards environment)
Along with Anil, a group of three to four students started performing the activity. They showed it to the rest of the class that solution turned green in colour on adding extract of China rose. Students performed the activity happily.
Lovely– Can we try with Periwinkle flowers?
Researcher – Please go ahead, you can try it, because researcher could see school garden was full of Periwinkle flowers. Children happily tried it.
“Can you now name the ash formed?”
Researcher gave them a hint-that it is _____oxide.
Students – Shouted, magnesium oxide.
They did not give her time to ask next question and said further, “on adding a few drops of water, it is now solution of magnesium hydroxide.”
Researcher invited one of the student to the blackboard and asked her to conclude what all they have learnt so far in the form of a word equation.
Researcher gave opportunity to children to ask any questions. She also told them to discuss among themselves. They started discussing and asking questions among themselves.
Group 1 to Group II –Name the natural indicator which we usually use in cooking and can also apply on our wounds if we get hurt?
Group II – Turmeric.
Group II to Group III – What will be the colour of turmeric solution on adding it to the solution of magnesium hydroxide?
Some turmeric powder was lying on table. Group III performed the activity and showed it turned red in colour.
Now they were so confident that they also tried nature of other metallic oxides, such as iron oxide by collecting rust from rusted articles.
Researcher told students to record their observations in the form of Table I.
Table I
S.No.Name of Metal Oxide
Change with Blue/Red Litmus paper
Change with China Rose Solution
Change with Periwinkle Flowers Solution
Change with Turmeric Solution
Children recorded their observations. This task helped her in assessing previous knowledge, experimental skills, observation, recording data, analysing data, providing explanation. She noticed how judiciously they were using materials. They washed, dried the apparatus and cleaned the table after the activity was over. She could also notice that some children were patiently doing the activities, other children were discussing with each other and everyone in the group wanted to try their hands at it.
Figure 4. Learning by doing
Researcher – “Do you like doing activities?”
Student’s responses–[To retain their original flavour, these are given in the original form].
- If we had understood the concepts given in class VII properly, we would not have faced difficulty in understanding in class VIII.
- Till date we used to think activities are given in the textbook just for the sake of giving only and these may not be feasible. Now we believe that we can perform them and understand better.
- When concepts are taught using black board and chalk only, we understand them superficially and remember them for short duration, but when we saw things happening in front of us, we remembered them forever.
Researcher could see a spark of satisfaction in their eyes and curiosity of learning more.
Example II - How to deal with difficult words?
While discussing chapter on “Acid, Bases and Salts” in Class VII (Textbook of Science, Class VII, 2008), children said that the following words are difficult for them to remember –
Phenolphthalein, Sodium hydroxide, Ammonium hydroxide, Potassium hydroxide, Magnesium hydroxide, Calcium hydroxide, Tartaric acid, Ascorbic acid, Oxalic acid, Acetic acid and please delete these words from this chapter.
Researcher told them “Nothing is difficult”.
Researcher– What is your name?
Student -Sunder
Researcher – What is your friend’s name, who sits next to you?
Student - Dinesh
Researcher – Whom do you like most in the class?
Student -Jaychand
Researcher – I have come to know that your sister is also studying in the same class. What is her name?
Student -Pooja.
Researcher – What is her best friend’s name?
Student - Monika.
Look, you have remembered so many names. Not only this, you know names of most of the students studying in this school. So remembering these words is not at all difficult. If we use these words frequently, we remember them forever.
So, today onwards, you will call me phenolphthalein.
Researcher – What will you call me?
Students – Phenolphthalein madam.
Researcher told Sunder – Your name will be Sodium hydroxide.
Jaychand - Your name will be Calcium hydroxide.
Pooja – We all will call you Ammonium hydroxide.
Researcher “re-named” for most of the students.
The next day onwards they greeted researcher “Good morning phenolphthalein madam” and their classmates with their given chemical names. This continued for a week.
Within a week’s time they remembered all the names of the chemicals and this method worked.
Example III - Freedom to explore
In class VI, researcher was discussing about “Solubility of Materials in Water” (Textbook of Science, Class VI, 2006).
Children are naturally curious; given freedom they interact and experiment with the things around them. Researcher gave freedom to children to try solubility of different materials in water. She told them to try with as many materials as they wanted.
They tried all sort of materials such as salt, sugar, sand, chalk powder, pieces of paper, turmeric powder, chili powder, wheat flour, gram flour, rice flour, cooked rice, green grass, sand, clay etc.
She showed them a chemical, copper sulphate or neelathotha (called in local language) and asked them – “Will it be soluble in water?”
Students –No!
Researcher – What makes you think that it will not be soluble in water?
Students – It looks like sand and we know sand is not soluble in water.
Researcher– Try it.
[A group of students while trying, as they dropped half a tea spoon of copper sulphate in a container containing water]
Rajkishore to other students – I told you it will sink.
Nisha – Starts stirring it with a spoon.
Neelam – Wow! It is dissolving.
Researcher – Can we get solid copper sulphate back from the solution?
Rajni – Yes.
Researcher – How?
Rajni – If we evaporate water, we can get back copper sulphate.
Rajni used her previous knowledge and could link concepts, which researcher had discussed earlier. Even class VII and VIII students said that copper sulphate will be insoluble in water.
However, at middle school level usage of copper sulphate is given in many activities in the textbooks. Unless and until children are not allowed to explore and perform activities, it will be difficult for them to understand. They were excited to see copper sulphate dissolving in water.
Example IV –Making teaching-learning interesting
A Crossword puzzle to be solved independently
Researcher engaged students in fun-filled and participatory form of teaching-learning process by giving cross-word puzzle. Students were excited to take up the challenges of filling up the crossword puzzle (Exemplar Problems in Science, Class VIII, 2012).This task helped researcher in enhancing student’s scientific vocabulary in an interesting manner (Koul, 2010).
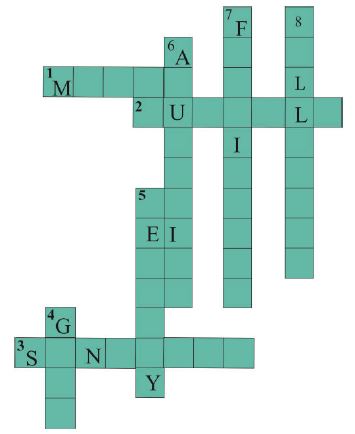
In Class VIII, researcher gave them the following crossword puzzle to solve individually.
Researcher drew puzzle grid on the blackboard and told children to draw on their notebooks. The crossword puzzle included clues based on the observation, classification, and application. She dictated the clues orally. This she did deliberately to check if children can write independently without any help. She wanted to strengthen their writing skills also. She gave them twenty minutes to solve this puzzle.
Complete the crossword (Figure5) with the help of the clues.
Figure 5. Crossword
Across
1. Which is generally hard, ductile, malleable and sonorous?
2. A metal is called so it can be drawn into wires.
3. Metal bells are used because of this property.
Down
4. A metal generally used for making jewellery.
5. A metal which is liquid at room temperature.
6. A metal which reacts with acid as well as base to form hydrogen gas.
7. Substances used to enhance the growth of plants.
8. Property by virtue of which metals can be beaten into thin sheets.
After 20 minutes, this crossword puzzle was discussed in the class. Most of them did clues (5) and (7) wrong. They did mistake in memory based clues. Some of them did spelling mistakes. Researcher corrected their mistakes and told them to write these spellings five times. This task helped her in assessing how good they were at thinking and working independently.
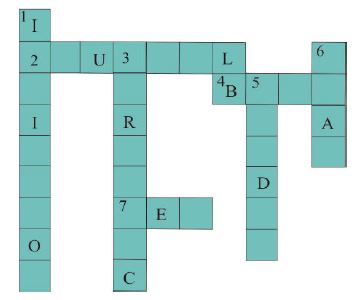
A Cross word puzzle to be solved collectively (Collaborative learning)
In Class VII following crossword puzzle from “Acid, Bases and Salts” was given. Researcher drew puzzle on the black board. This time she told them to solve this puzzle orally and collectively. Studies have shown that children learn better when they work in groups (Ginsburg Block, Rohrbeck&Fantuzzo, 2006; Johnson & Johnson, 1989). She divided the class into three teams A, B and C. In order to save time, she utilised the way students were sitting in the class in three different rows to make heterogeneous groups. This was a group activity. Children were answering after discussing in their groups. She encouraged them to help each other in looking for an answer. She could notice that some children were patiently listening to others.
Fill in the crossword (Figure 6) with the help of the clues provided.
Figure 6. Crossword
Across
(2) The solution which does not change the colour of either red or blue litmus.
(4) Phenolphthalein gives pink colour in this type of solution.
(7) Colour of blue litmus in lemon juice.
Down
(1) It is used to test whether a substance is acidic or basic.
(3) It is a natural indicator and gives pink colour in basic solution.
(5) Nature of ant’s sting.
(6) It is responsible for increase in temperature during a neutralisation reaction.
Most of the children did clues (2) and (6) wrong. Researcher noticed that children have not understood the concept of neutralization. She made them repeat the activity, and children performed the activity step by step by raising and discussing number of questions. This time they were successful in understanding the concept
Example V - Quiz in a collaborative way
A quiz was organized in Class VII (Exemplar Problems in Science, Class VII, 2012), where researcher ensured participation of all the students. She divided the class into three groups (6 children in each group). With this activity she could test children’s mental ability, awareness and speed with which they can recall the information.
Researcher posed questions where emphasis was laid on the learning processes of science. These include observations, hypothesis, measurement of data, collection, analysis, drawing inferences, making generalisations and promoting creativity.
The following questions were posed to the students.
1. The correct way of making a solution of acid in water is to
(a) Add water to acid. (c) Mix acid and water simultaneously.
(b) Add acid to water. (d) Add water to acid in a shallow container.
2. Products of a neutralization reaction are always
(a) An acid and a base. (c) A salt and water.
(b) An acid and a salt. (d) A salt and a base.
3. Turmeric is a natural indicator. On adding its paste to acid and base separately, which colours would be observed
(a) Yellow in both acid and base. (c) Pink in acid and yellow in base.
(b) Yellow in acid and red in base. (d) Red in acid and blue in base.
4. Phenolphthalein is a synthetic indicator and its colour in acidic and basic solutions, respectively are
(a) Red and blue. (c) Pink and colourless.
(b) Blue and red. (d) Colourless and pink.
5. When the soil is too basic, plants do not grow well in it. To improve its quality what must be added to the soil?
(a) Organic matter (c) Slaked lime
(b) Quick lime (d) Calamine solution
6. A solution changes the colour of turmeric indicator from yellow to red. The solution is
(a) basic. (c) neutral.
(b) acidic. (d) either neutral or acidic.
7. On adding phenolphthalein indicator to a colourless solution, no change is observed. What is the nature of this solution?
(a) Basic (c) Either acidic or neutral
(b) Either acidic or basic (d) Either basic or neutral
8. Which of the following is an acid-base indicator?
(a) Vinegar (c) Turmeric
(b) Lime water (d) Baking soda
9. Look at the given reaction. Hydrochloric acid on reacting with sodium hydroxide gives sodium chloride and Water. Sodium chloride formed in this reaction remains in solution form. Can we get solid sodium chloride from this solution? Suggest a method.
State whether the following statements are true or false. Correct the false statements.
10. All substances are either acidic or basic.
11. A compound if acidic will turn all indicators red.
12. Common salt dissolved in water turns blue litmus red.
13. Calamine can be used to treat ant’s sting.
14. While playing in a park, a child was stung by a wasp. Some elders suggested applying paste of baking soda and others lemon juice as remedy. Which remedy do you think is appropriate and why?
None of the group could answer question 4 and 7. To make children understand the questions 4, researcher told children to repeat the activity. They took lemon juice and soap solution in two different test tubes and poured a drop of phenolphthalein solution in both the test tubes. They could see soap solution turned red whereas there was no change in the colour of the lemon juice. For question 7, children took acidic, basic and neutral solutions in three separate test tubes and added a drop of phenolphthalein solution in each of the test tube. They could observe only basic solution turned pink in colour where as in acidic and neutral solution there was no change in colour. Children were satisfied after performing the activities.
In Class VI, a quiz was conducted on chapter “Sorting Materials into Groups”. In this class researcher did quizzing in a slightly different way. She divided the class in three groups A, B and C. She initiated the quiz and subsequently questions were to be asked by the students. She asked first question to team A, which was answered by team A. Then team A framed a question for team B. Team B answered the question and framed a question for Team C. In this way questions and answers were circulated around all the teams and with this play way method entire chapter was revised and children also developed the skill of posing questions.
A sample of some questions which were raised by students are given here, without any modification.
- Is chalk powder soluble in water or not?
- Will glass tumbler sink in water or float?
- Find odd one out from the following: Key, stone, sand and oil
This was an interesting way of revising the chapter and children used to have lots of fun. The moment researcher used to complete any chapter, children would say “Let up play quiz?” She noticed, it was easier for children to learn, remember and understand by involving all the children of the class. If joyful environment is created in the classroom, children learn more and never feel bored.
Example VI - Collaborative project work
In a project work children get an opportunity to identify a problem, design a work plan to address the problem, look for appropriate resources, carryout the plan, record the data/information and draw conclusions on the basis of recorded data. It has been seen that children with low to average verbal ability and children with little previous content knowledge learn more through project based learning classes than in traditional classes (Mergendoller, Maxwell &Bellisimo, 2006; Mioduser & Betzer, 2003). Project work developed self-confidence and independent thinking among children.
Some of the projects which children did are highlighted below.
- Using the knowledge of acids and bases, they wrote secret messages and made greeting cards with the help of soap solution and turmeric.
Figure 7. Greeting card
- Using natural indicators such as flowers of periwinkle, rose, China rose, they tested nature of number of materials as acidic, basic or neutral
- Brought samples of water from nearby areas and tested the nature of these water samples (acidic, basic or neutral).
- Essential conditions for rusting of iron and in which bottle evaporation of water was maximum (because in one of the bottles they poured hot water to remove the dissolved oxygen and on top of it they poured a layer of oil).
Each group presented their study. Questions were asked by other groups. There was a lively atmosphere in the class. Everyone was free to throw out their ideas without any fear. Researcher noticed the growing confidence among children. Most of them could communicate their observation and conclusions and could read and write without any hesitation. However, a few of them were good at communication but poor at writing and reading. Researcher had to spend extra time with these children on their writing and reading. Whenever researcher had to write anything general or related to concept on the black board, deliberately she would request these children to do so. Children love to write on the blackboard. This practice helped in gaining writing confidence among these children.
Example VII - Written test
Teachers will never let go children without written test!
In a written test teachers usually frame a set of questions to assess the extent of learning, in a given content area, the child has attained (Koul, 2008). Children are usually graded according to the performance. The outcome of the written test is used to improve the teaching-learning process.
When researcher entered Class VIII, she was told that children are through with the chapter on “Metals and Non-metals”. To assess the extent of learning in this chapter, she gave them the following pre-test.
Pre-test
1. Which of the following is not a metal?
a. Copper
b. Sulphur
c. Aluminium
d. Iron
2. The substance that will be flattened on being beaten with a hammer is
a. crystal of iodine
b. lump of sulphur
c. piece of coal
d. zinc granule
3. Materials which can be drawn into wires are called ductile. Which of the following is not a ductile material?
a. Sodium
b. Copper
c. Sulphur
d. Aluminium
4. Generally metallic oxides are basic and non-metallic oxides are acidic in nature. Solution of which of the following oxides in water will change the colour of blue litmus to red?
a. Sulphur dioxide
b. Magnesium oxide
c. Iron oxide
d. Copper oxide
5. Nita prepared a blue coloured solution of copper sulphate in beaker A and placed an iron nail in it. Nancy prepared a bluish green solution of ferrous sulphate in beaker B and placed a copper wire in it. What changes will they observe in the two beakers after an hour?
Out of 17 students (one was absent) only three children answered questions 2 correctly. Question 5 was answered correctly only by one student.
The researcher provided crystals of iodine, lumps of sulphur, pieces of coal and some zinc granules to children and told them to check which one of the materials get flattened by beating with hammer. By performing this, they got sure that only zinc granules got flattened, rest all other materials were brittle.
For question 5, the researcher told children to repeat activity of displacement reaction. They took solutions of copper sulphate and ferrous sulphate in two separate beakers and dropped an iron nail in copper sulphate solution and copper wire in ferrous sulphate solution. After half an hour they observed copper was displaced by iron from copper sulphate solution. Whereas nothing had happened in ferrous sulphate solution in which copper wire was dropped.
The researcher discussed this chapter not only by doing activities but also giving other tasks to children such as crossword puzzle, projects, quiz, assignments etc. She gave freedom to children to explore, observe, and perform all the activities by themselves in groups. Children enjoyed learning by doing and discussing in groups. Then on their demand, researcher gave them a post-test.
Post-test
1. Metals generally react with dilute acids to produce hydrogen gas. Which one of the following metals does not react with dilute hydrochloric acid?
a. Magnesium
b. Aluminium
c. Iron
d. Copper
2. Which of the following non-metals reacts and catches fire on exposure to air?
a. Phosphorus
b. Nitrogen
c. Sulphur
d. Hydrogen
3. Generally metallic oxides are basic and non-metallic oxides are acidic in nature. The solution of which of the following oxides in water will change the colour of blue litmus to red?
a. Sulphur dioxide
b. Magnesium oxide
c. Iron oxide
d. Copper oxide
4. Which of the following reacts with cold water vigorously?
a. Carbon
b. Sodium
c. Magnesium
d. Sulphur
5. Which of the following property is not responsible for copper to be used as electrical conduction wires?
a. Ductility
b. Colour
c. Good conductor of electricity
d. It is solid
6. Zinc sulphate forms a colourless solution in water. Will you observe any colour on adding copper turning in it?
7. Rima prepared a blue coloured solution of copper sulphate in beaker A and placed a copper wire in it. Rahul prepared a bluish green solution of ferrous sulphate in beaker B and placed an iron nail in it. What changes will they observe in the two beakers after an hour?
All the 17 children did question 1 wrong. Almost 10 children attempted questions 6 and 7 wrong. Researcher noticed that children have not understood the concepts of displacement. Initially she was little depressed but she did not lose hope and she explained these concepts by demonstrating the activities again by involving children and she was bound to discuss about activity series, which actually has been explained at secondary stage. Children who had understood the concept better, she told them to explain it to others. This helped her to certain extent. Her observation is that children of class VIII find concept of displacement reaction difficult because textbook do not talk about the activity series at this stage.
Example VIII - Maintenance of portfolio
The researcher wanted to have evidence of every child’s knowledge, skills and attitudes. To document children’s growth, she told them to maintain their portfolios (Source Book on Assessment in Science, 2012) on whatever tasks they did.
Since these children were from economically weaker section of the society and were not in a position to buy files, she gave them newspapers and told them to fold the newspaper in the form of a file. Paste a blank paper over it and write down your name and class. She was surprised to see the way children decorated their files (portfolios). Whatever work she used to assign them, they used to complete it, show it to her and add it to their portfolios.
Figure 8. Portfolio
Portfolios of the children helped her in the following ways:
- Children could reflect and assessed their own work for improvement.
- Due to these portfolios researcher’s communication with students, teachers were more frequent and fruitful. She could communicate students about their performance.
- She could assess their ideas, interest, learning styles, process skills, inquiry skills, content knowledge etc.
- She could know the child holistically.
Some children carried portfolios every day in their bags and some used to keep them in the class cupboard.