Asia-Pacific Forum
on Science Learning and Teaching, Volume 12, Issue 2, Article 9 (Dec., 2011) |
35 Form Four students were selected as the sample for the pilot study in order to investigate the effect of EC Lab on the students’ knowledge and motivation in the learning of Electrochemistry. The sample consisted of 13 male and 22 female students (aged 16 years), and the majority of them are Malays.
Materials utilized in the study are the specific entry test, pretest, post-test, motivation questionnaire and the IMMPA titled EC Lab. The specific entry test consists of ten multiple choice questions testing on some basic skills that will be applied in the learning of Electrochemistry. The purpose of the specific entry test is to identify students’ specific entry competencies related to proton number, nucleon number, arrangement of electrons, chemical formulae and chemical equation. Students were given the chance to recall their prior knowledge on the related basic skills before they start the intervention. Achievement tests were administrated in the form of pretest and post-test before and after the intervention. There are two structured questions in the achievement test. The questions test knowledge on electrolytic cell and voltaic cell concepts at the macroscopic, microscopic and symbolic levels. Macroscopically, the students need to identify the anode and cathode in the cell and describe the observations at both electrodes during the electrolysis process. Microscopically, they need to draw the ions that exist in the electrolyte and the direction of the flow of the electrons in the circuit. Symbolically, they have to represent the oxidation and reduction process at the electrodes by writing the half-equations. Questions in the pretest and post-test are similar in terms of difficulty level and concepts tested. The only difference is the types of electrodes and electrolyte used in the cells. Reliability analysis was carried out and the Kuder Richardson KR-21 reliability index is 0.65 for the pretest and 0.71 for posttest.
The motivation questionnaire is a Likert scale questionnaire. There are three sub dimensions involved, namely Adhered Value, Expectancy Components and Affective Components. Adhered Value consists of three constructs, namely intrinsic goal orientation, extrinsic goal orientation and task value. On the other hand, Expectancy Components consist of control of learning belief construct and self-efficacy for learning and performance construct. Affective Components involve test anxiety construct. There are 28 items in the questionnaire with Likert scale provided, where 1 – Strongly Disagree, 2 – Disagree, 3 – Not Sure, 4 – Agree, and 5 – Strongly Agree. Items in the questionnaire have been taken from the study of Sadiah and colleagues (2009) which were translated from the instrument used by Pintrich and DeGroot (1990). In this study, the researcher used the motivation section only and changed the scale from seven points to five points. The Cronbach Alpha for the motivation questionnaire is 0.87.
EC Lab was developed by the researcher by using the combination of two instructional design models: the Kemp Model and Gerlach and Ely Model. The reasons for using the combination of these two models are because they are classroom-oriented models (Gustafson & Branch, 1997) with their own strengths. The Kemp Model describes elements, not ‘step, stage, level or sequential item’ in an instructional design (Kemp et al., 2004). The oval shape of the model indicates the independency of the elements in the model. It is a non-linear model with no starting and ending point. All the processes of designing, developing, implementing and evaluating can be done concurrently and continuously. The Gerlach and Ely Model is suitable for the novice instructional designers who have knowledge and expertise in a specific context (Qureshi, 2001, 2003, 2004). This model is classroom-oriented and is suitable for teachers at secondary schools and higher education institutions. The Gerlach and Ely Model focus more on the instructional materials and resources without identifying the instructional problems. Hence, the researcher combined the two models as the instructional design model to develop the EC Lab. The conceptual framework of the combination of these two models used in the study is presented in the Appendix.
There are two PAs in the EC Lab, namely Professor T and Lisa. Professor T is a sixty year-old male PA who acts as an expert in Electrochemistry. He gives accurate information and explains new concepts to the students. Professor T speaks slowly in a formal way with little body gestures and facial expressions. On the other hand, Lisa is a fifteen-year old female youth who speaks with an energetic voice. She is a learning companion in the EC Lab. She learns together with the students, gives motivation and encouragement to the students to complete the tasks and exercises in the module. Students are free to choose the PA they want to accompany them in the learning of Electrochemistry when using the EC Lab.
The main menu for the EC Lab consists of tutorial, experiment, exercise, quiz, memo and game. There are five sub units in the EC Lab: (1) Electrolytes and Non-Electrolytes, (2) Electrolysis of Molten Compounds, (3) Electrolysis of Aqueous Solutions, (4) Voltaic Cells and (5) Types of Voltaic Cells. All the information delivery for the sub units are presented in the tutorial session. The experiment session consists of five experiments in Electrochemistry. The first experiment about the concept of electrolyte and non-electrolyte is done through the application of simulation. Another three experiments investigating the factors that determine the ions to be discharged at the electrodes and experiment for simple voltaic cell are hands-on investigation. The students are guided by the PAs to carry out the experiments in the chemistry laboratory and they need to apply scientific process skills and manipulative skills in order to carry out the investigations. After the information delivery process, the students will do some exercises to enhance their understanding on the concepts learnt. A quiz will be given at the end of every sub unit. Each quiz is divided into three levels. The first level is to let the students do some reflections on what they have learnt in the sub unit. The students then need to compare their prior idea with the new idea to review whether the conceptual change has occurred. The second level of the quiz consists of five simple multiple choice questions and some elementary structured questions. The third level of the quiz is more challenging with some difficult structured questions and essays. Memo is created to give some hints and tips on learning of some of the Electrochemistry concepts. For instance, mnemonics are given to help the students in memorizing the list of anions and cations in the Electrochemical Series. There are four activities in the game session to let the students relax their mind after the learning process. The activities are applications of Electrochemistry concepts; for instance, one of the activities asks the students to set up an electrolytic cell and a voltaic cell with the apparatus given.
The complete flow of each sub unit follows the five phases in the learning process created by Needham (1987). The five phases are orientation, elicitation of ideas, restructuring of ideas, application of ideas and review. In the EC Lab, the Think About It session (Figure 1) is the orientation phase. The students will be shown some pictures that are familiar to them. Those pictures are related to the concepts to be learnt in every sub unit.
Figure 1. Think about it session in orientation phase
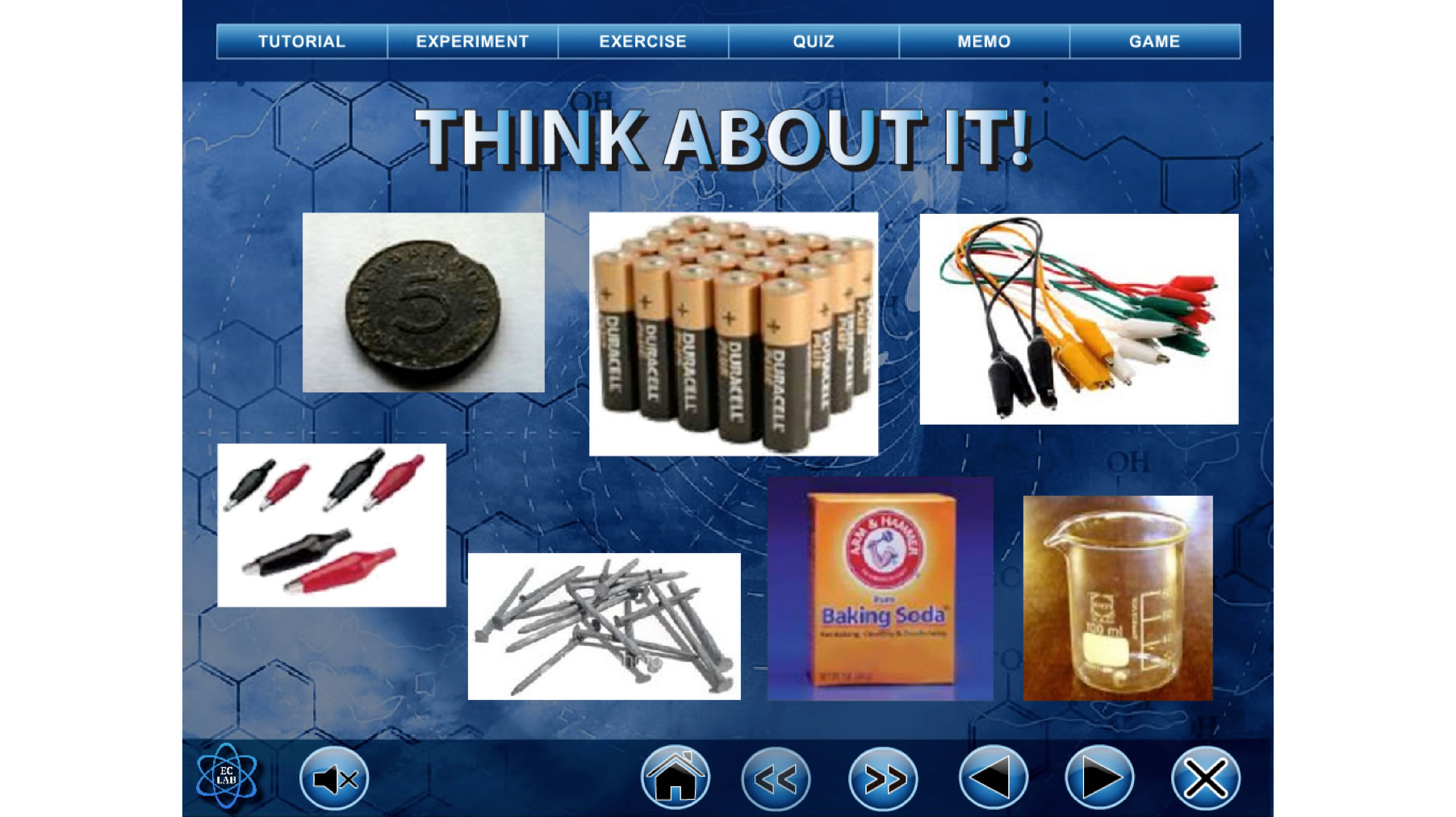
Then, in the Do You Still Remember? session, the students will be reminded of some concepts that they have learnt before. Those concepts are related to the new concepts to be learnt in the sub unit. Next, in the Give Me Your Ideas session, the students are given the chance to give their ideas regarding some activities that are related to the concepts to be learnt. Then, in the Are You Sure? session, the students need to give some ideas, make some guesses or predictions on some outcomes of the situations. In order to examine their ideas, guesses and predictions, the students need to carry out some investigations in Let’s Do It (Figure 2) or watch related videos in Show Time sessions. In these two sessions, the students will be exposed to the conflict situations if their ideas, guesses or predictions are different from what is being shown in the experiments or videos. Hence, conceptual change should happen here and the students need to modify, extend or replace their existing ideas.
Figure 2. Simulation activity in Let’s do it session
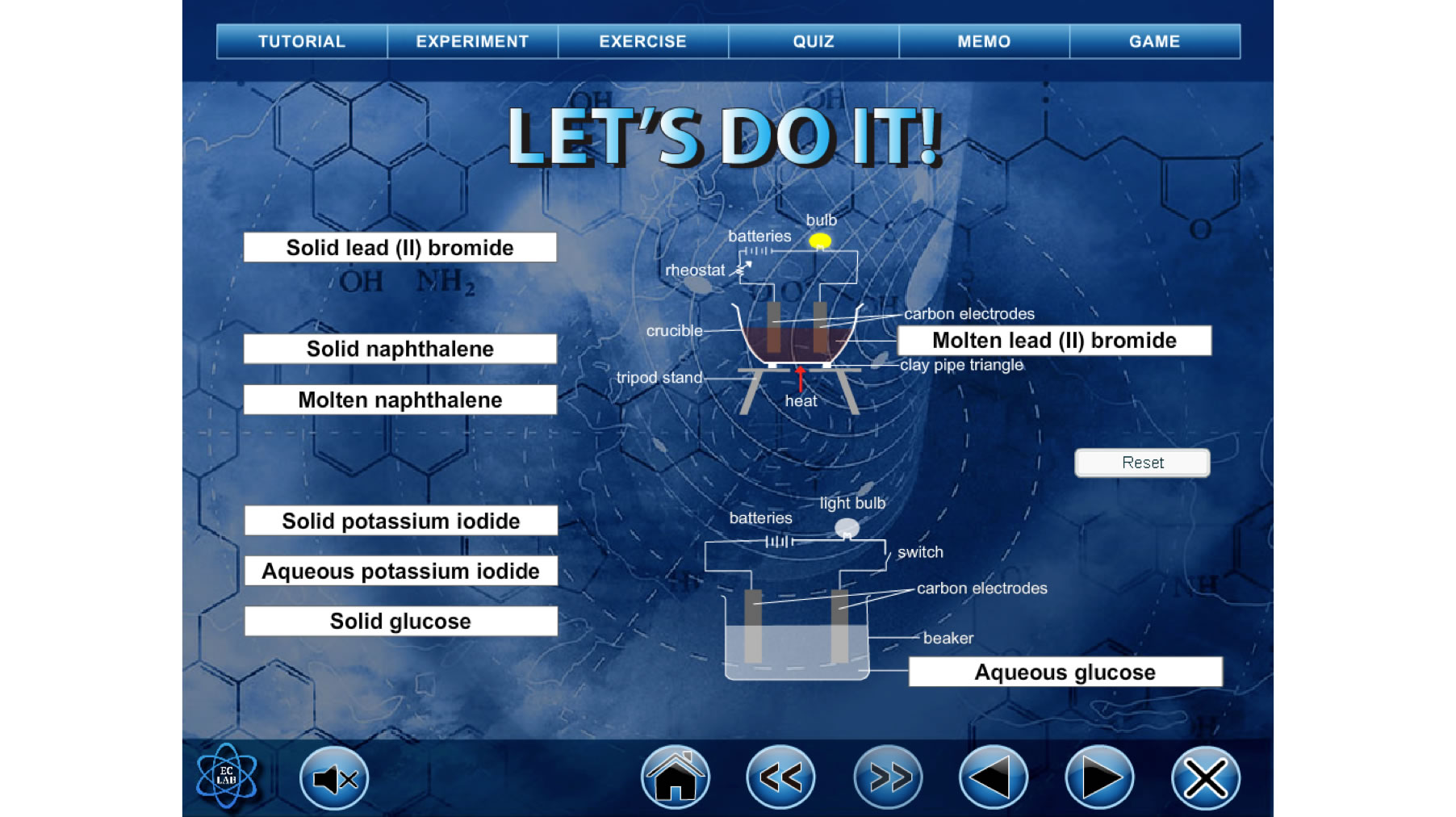
Then, reinforcement of the constructed ideas will be done in the Practice Makes Perfect session. The students will apply the concepts learnt in new situations and examples. Lastly, Before and After session (Figure 3) is created to enable the students to reflect upon the extent to which their ideas have changed. The students need to answer certain activity questions again and compare their prior answers to the new answers. Testing Yourself and Challenge Yourself sessions contain multiple choice questions, structured questions and essay questions to let the students evaluate themselves on the concepts learnt.
Figure 3. Before & after session in review phaseThe samples were given the specific entry test, pretest and motivation questionnaire to study their existing knowledge in Electrochemistry and their motivation level in studying Chemistry. The students were given 80 minutes to answer the specific entry test, pretest and motivation questionnaire. The students who had poor results for the specific entry test were given some revision notes. They were told to study the revision notes before the treatment sessions. The second meeting was carried out after the school session where the students were gathered at the computer laboratory. Students need to put on the earphone to listen to the script delivered by the PAs. The user manual was given to the students, followed by a briefing on how to use the EC Lab. Then, students were free to explore the first and second sub unit in 160 minutes. The third and forth meeting were conducted at the chemistry laboratory to carry out the experiments investigating the factors that determine the ions to be discharged at the electrodes for the third sub unit. The principal of the school limited the duration of the pilot study to four meetings; hence, the students only studied three sub units in the EC Lab. They only studied the concept of electrolytic cell. After the investigations, the students were given the post-test and motivation questionnaire, and they were asked to answer these instruments in 80 minutes.
The specific entry test, pretest and post-test were marked according to the answer scheme developed. Each correct answer was given one point while the wrong answer was given zero point. Then, the pre and post motivation questionnaires were analyzed by using SPSS version 18.0 to find out the mean values for each construct. Paired-sample t-test was conducted to evaluate the impact of the EC Lab on the students’ achievement test and motivation level.