|
Asia-Pacific Forum on Science Learning and Teaching, Volume 7, Issue 1, Article 6 (June, 2006) Hong Kwen BOO Primary science assessment item setters' misconceptions concerning the state changes of water
|

|
Asia-Pacific Forum on Science Learning and Teaching, Volume 7, Issue 1, Article 6 (June, 2006) Hong Kwen BOO Primary science assessment item setters' misconceptions concerning the state changes of water
|
Misconceptions Concerning State Changes of Water
In Singapore, the subject of state changes and related heat transfers is introduced to pupils at the P4 level. The key concepts taught are the three states of matter, the state changes of freezing/melting and boiling/evaporation/condensation with understanding of the differentiation between evaporation and boiling.
The concepts involved are a recurrent source of problems to many question setters as illustrated by the following test items.
In example question 1 which contains three generic statements about ice and water, the intended answer is option (4) indicating that all three statements given about water and ice are true.
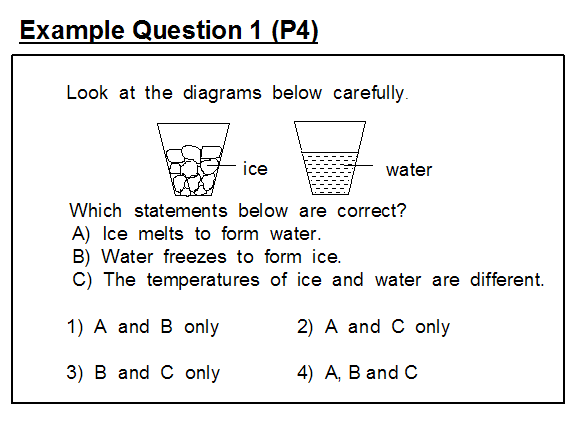
However, statement C is problematic since ice and water can both exist at 0°C. Pupils should have been taught that the temperature of ice, for example brought out of the freezer at (say) -10°C, will gradually rise until it reaches the melting point, 0°C, and then remain constant until all of the ice has melted after which the temperature of the water will begin to rise until it attains the ambient temperature.
This item was improved by re-wording statement (C) as:
(C) The temperature of ice and water are always different and then making option (1) the answer key.
Example question 2 1 shows the same misconception in terms of the process of freezing.
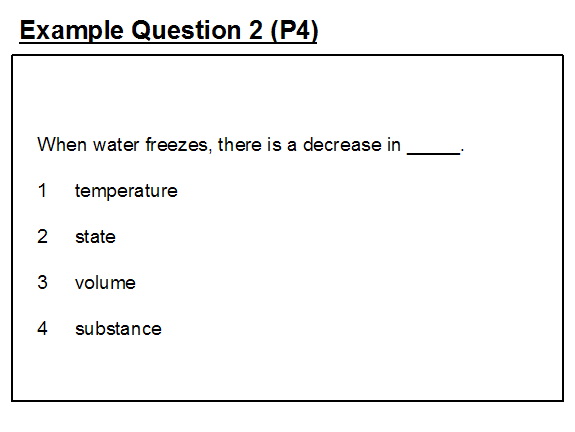
Again, pupils will have been taught that when water changes to ice, the temperature remains constant at 0°C until all of the water has turned to ice. It may be that in, crafting the question, the question setter is thinking at an ‘everyday’ level of filling an ice cube tray with water from the tap at 25°C (in Singapore), putting it in the freezer and then the following morning taking out ice cubes at -10°C. It is important that pupils understand that, in science, words have a very precise meaning and that meaning may be different to the generally used meaning in everyday circumstances. In science, ‘freezing’ refers specifically to the changing of liquid to solid at the freezing point temperature.
Whilst example 2 may have been a matter of imprecise item crafting, the same cannot be said of example 3 where the question setter's understanding is explicitly indicated by intended answer of option (2) showing a fairly constant rate of temperature decrease throughout the time the beaker of water is left in the freezer and changed to ice.
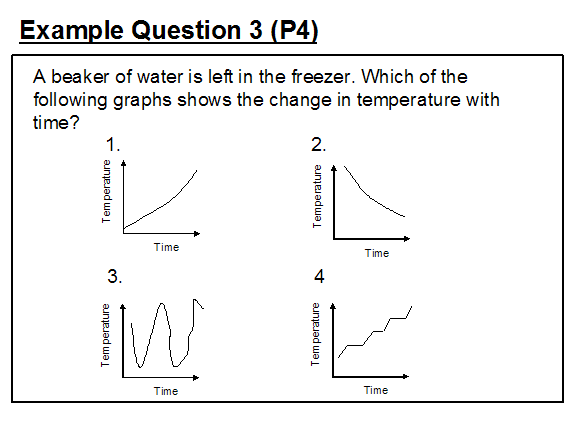
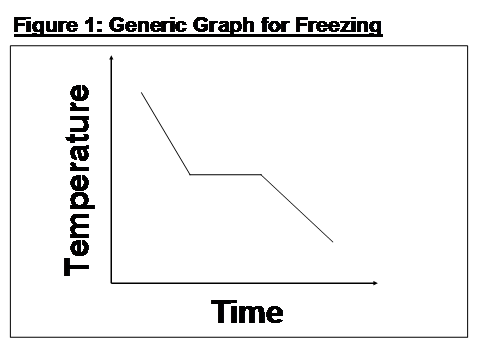
Whilst, at the primary level, pupils are not taught the detail of latent heat, they are provided with the understanding that temperature remains constant whilst freezing takes place and should have seen the correct generic graph illustrated in Figure 1.
In example question 4 we see the question setter exhibiting a very narrow understanding of the range of changes that are taking place with water at 0°C.
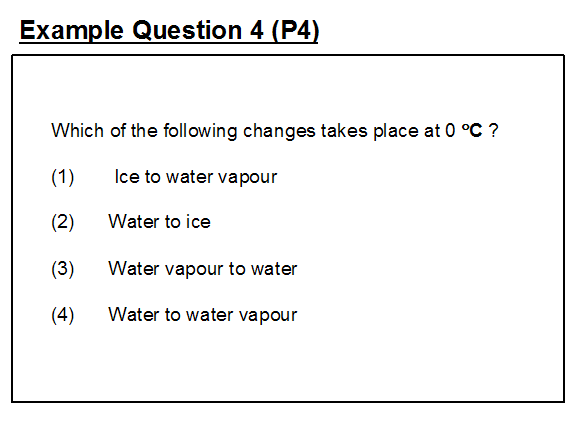
The intended answer is option (2) indicating that, as far as the question setter is concerned, freezing is the only change that might be taking place. In fact options (2), (3) and (4) are all correct 2. Many question setters have the misconception that some minimum temperature is required before evaporation can take place. In fact, evaporation will take place from the surface of a liquid at all temperatures in the liquid phase (Option 4). The effect of temperature is to affect the rate at which evaporation will take place. Evaporation occurs even when liquid water is at 0°C. Water vapour can condense at any temperature, even when the water vapour is at 0°C; hence option (3) is also a possible answer.
Example questions 5 and 6 illustrate similar misconceptions and item crafting problems concerning the process of boiling. The question setter's intended answer to question 5 is option (1). By including statement D, the question setter is exhibiting the misconception that steam and water are at different temperatures. However, when water boils at 100°C, the steam produced is also at 100°C 3.
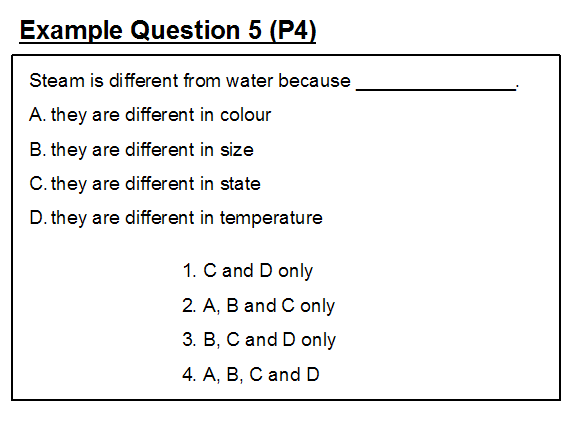
In this question, pupils may have difficulty with not including statement B in their answer having been taught that liquids have a definite volume whereas gases do not .
Example question 6, where the intended answer is option (2), shows a similar misconception to the one seen in questions 2 and 3: the misconception that state change is always accompanied by temperature change; in this case, when a liquid boils there is a rise in temperature.
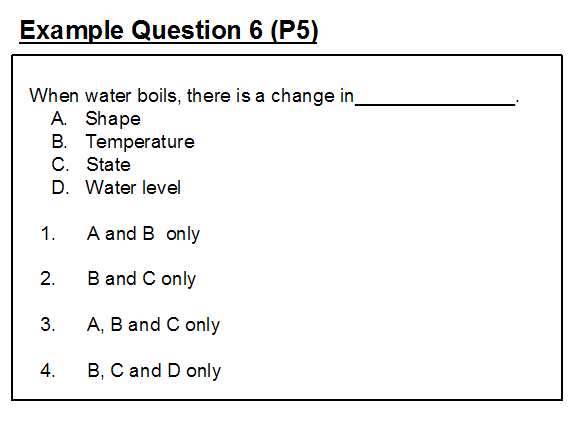
The intended answers suggests a conception that, at the boiling point, the water is at 100°C whilst the steam produced is at a temperature greater than 100°C. However, as with question 2, the problem may be one of item crafting and the dominance of everyday thinking in the use of language. When we say “boil the water to make some coffee” what we are describing is the process of raising the temperature of the water from room temperature to boiling point. If we then continue to apply heat, for example by not switching off the heat source, then the water will boil and steam will be produced. It is important to emphasise that when a scientist talks of boiling he specifically means the change from liquid to gas at the boiling point temperature. Steam, if contained, can be further heated to even higher temperatures.
Many question setters are unclear about the precise details associated with adding a solute to ice or an ice/water mixture.
Example question 7 illustrates a typical question setter's misconception on this matter.
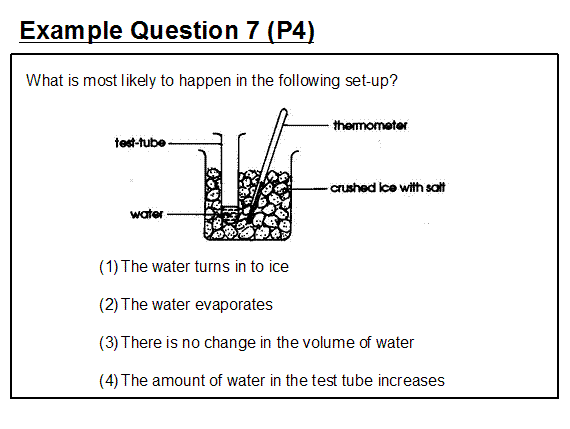
In this example, option (1) is the intended answer. However, in practice this outcome may not occur. It all depends upon the amount of salt added to the crushed ice. The misconception is that any amount of salt will sufficiently lower the temperature of the crushed ice to cause the water in the test tube to freeze. In fact, the desired effect is related to the mole fraction (amount) of added salt up to the eutectic point. The origin of this misconception may be the result of teachers simply repeating a workbook experiment with a single quantity of salt and observing a lowering of the freezing point of the ice and then not experimenting further with different amounts of salt to reveal the cause-effect relationship between the amount of salt added and the resultant freezing point of the ice-salt mixture. Whilst the relationship between the amount of salt added and the lowering of the melting point of ice and the term eutectic point are not explicitly introduced in the primary syllabus, it is important that teachers avoid introducing alternative conceptions at this level since these could form resistant barriers to further learning at a later stage. Of the options provided, option (2) is the only one that we can be sure will take place whatever the amount of salt added to the crushed ice. This was certainly not the intended answer that the question setter had in mind and further illustrates the misconception concerning evaporation discussed under Example Question 4 that some minimum temperature is required before evaporation takes place. A further problem with the question as presented is that the quantity of ice may be high enough and/or its temperature low enough to cause freezing of the water in the tube even without the addition of salt.
A variant of the misconception concerning the addition of salt to ice is shown in example question 8.
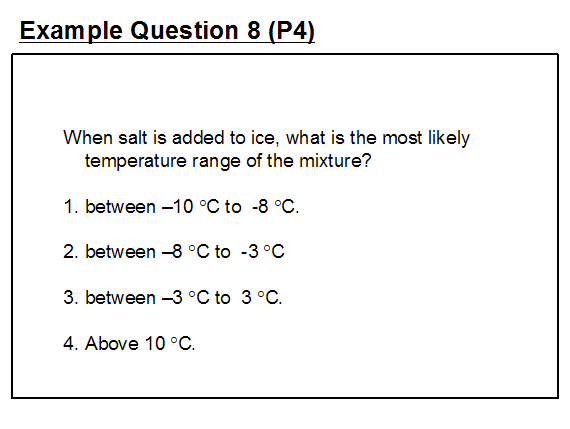
The setter's intended answer is option 1 indicating the misconception that the decrease in the temperature of the ice is independent of the amount of salt added – i.e. that any amount of salt, however small or large when added to ice will produce this very narrow temperature range. In fact, depending upon the amount of salt added the temperature could fall to as low as -20°C. This question would be better re-crafted as a free response item asking pupils to describe the effect of adding salt to crushed ice.
1 The question need not refer to pressure since the effects of pressure on state changes are outside the scope of the current Singapore primary science syllabus
2 Whilst Option 1 is also true (sublimation also occurs), the concept of sublimation is outside the scope of the current Singapore primary science syllabus.
3 The effects of pressure on state changes are outside the scope of the current Singapore primary science syllabus.
Copyright (C) 2006 HKIEd APFSLT. Volume 7, Issue 1, Article 6 (June, 2006). All Rights Reserved.