Asia-Pacific Forum on Science Learning and Teaching, Volume 17, Issue 2, Article 5 (Dec., 2016) |
The results obtained from the study were evaluated in accordance with the research questions. Prior to gain score analysis, an independent sample t test analysis was used to compare the mean differences between the experimental and control group with regard to the students’ pre-test scores on their understanding of the electrochemistry test. Results of the independent sample t-test analyses showed that there was a significant difference between the experimental group (M = 6.82, SD = 2.89) and control group (M = 4.53, SD = 2.76; t (113) =4.36, p=0.000, two-tailed). The magnitude of the differences in the means was large (eta squared = .144). Therefore, the confounding effect of students’ pre-test scores should be controlled in the following analyses. In order to control the confounding effect of students’ pre-test scores, gain scores were used to examine the effect of the teaching method.
Comparison of Gain Scores of Students (Results for First Research Question)
In order to solve the first research problem, the independent samples t-test analysis was performed to compare the means of the gain scores obtained from the difference between the students’ pre- and post-test scores. The Levene’s test results were checked and it was found that the significance level was greater than p= .05, and that the assumption of equal variance was satisfied. The independent samples t-test results showed that there was a significant mean difference between the experimental and control group students with regard to the means of their gain scores, t(113)=5.65, p<0.01. The experimental group students’ gain score average (M=7.48), was higher than those of control group students (M=3.98). The eta square value calculated was η2=.22. Accordingly, the magnitude of the differences in the means of gain scores was quite high (see Table 1).
Table 1. Independent Samples t-test Results
Group
N
Mean
Std. Deviation
Levene’s Test
t
df
Sig.
F
Sig.
Gain Score
Experimental
56
7.48
3.09
.65
.42
5.65
113
.000
Control
59
3.98
3.53
Students’ Levels of Conceptual Understanding (Results for Second Research Question)
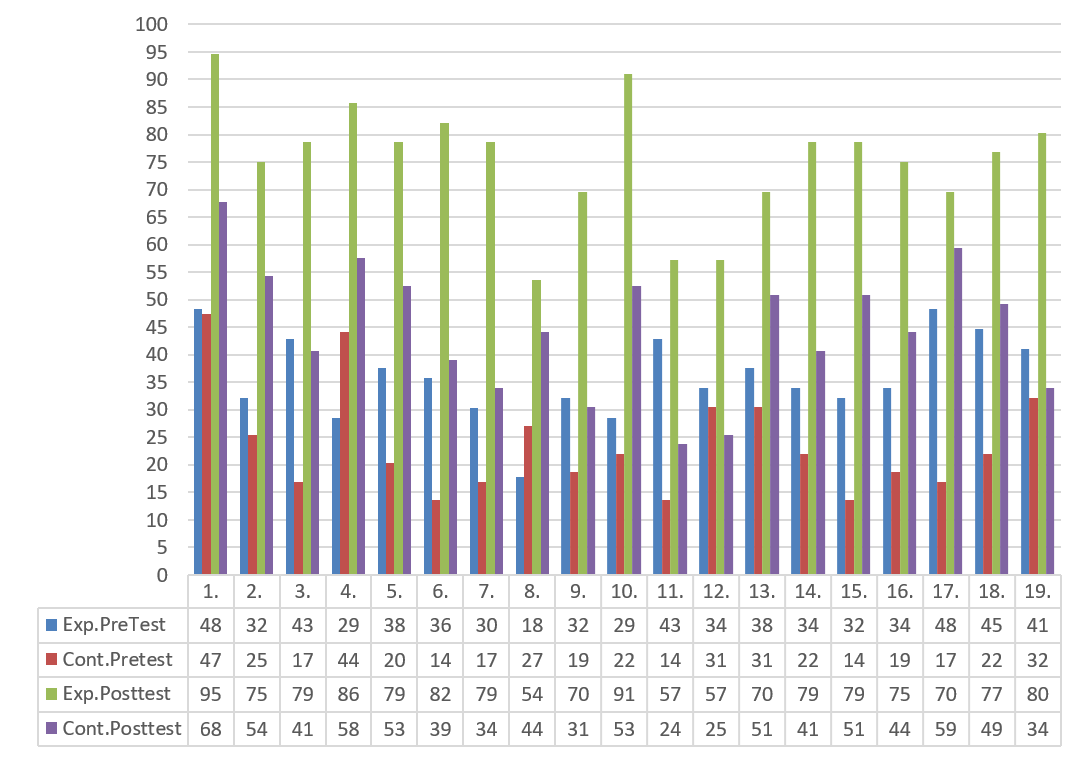
Students’ ECT post-test answers were examined at the end of the application, so as to check the experimental and control group students’ understanding of electrochemistry in relation to a solution for the second research problem. In consequence, the rates of the ECT post-test correct answers of the experimental and control group students were compared, and the findings were shown in Figure 2. An examination of the percentages given in Figure 2 makes it clear that the percentage of experimental group students ‘correct answers to questions 8, 11 and 12 in the ECT post-test is below 70%. The students in the experimental group had the highest rate of mistakes in these three questions. Therefore, these questions were examined. Appendix B shows the percentage of students’ answers to a sample question in the test.
Figure 2. A comparison of the rates of experimental and control group students’ correct answers
On examining the questions in ECT, it was found that students had various alternative conceptions (Table 2). In question 8, an attempt was made determine whether or not students had alternative conceptions as “the potential of standard hydrogen electrode is found as a result of experimental procedures”. While 57.14% of the students in the experimental group responded correctly to the first two stages of this question, 54% said that they were not sure of their answers. On examining the answers given by the students in the control group, the rate was found to fall from 45.76% to 44%. To the statement “that standard electrode potentials for H2(1atm)/H+(1M) is zero was found in consequence of experimental measurements”, 73.21% of the experimental group students and 57.63% of the control group students gave correct answers. It was found in the post-test that 10.71% of the experimental group students and 16.95% of the control group students had an alternative conception as “standard hydrogen electrode was chosen as the standard reference electrode, and standard electrode potentials of this electrode at 298,15 K (25 °C) was regarded as (E°) 0,0000 V”. Besides, 71.4% of the experimental group students and 13.56% of the control group students had alternative conceptions as “Mg2+(aq) + 2e- → Mg(s) reduction half-equations occur in cathode, and H2(g) → 2H+ + 2e- oxidation half equations occur in anode”; while 10.71% of the experimental group students and 6.78% of the control group students had alternative conceptions as “E0 value of H2(1 atm)/H+(1 M) is zero and this is derived from the chemistry of H+ and H2”. One of the distractors used in this question, the alternative stating “since half-cell potentials are the values which are obtained by measuring, they are precise and are used in calculating half-cells” was chosen by 7.14% of the students in the experimental group and 11.86% of the students in the control group. At the second stage of the question, the proportion of correct answers was 64.29% in the experimental group, and 50.85% in the control group.
In question 11, an attempt was made to determine whether or not students had alternative conceptions about concentration cells. 60.71% of the experimental group students answered the first stage of this question correctly while 57% of the students said that they were sure of their answers to the first two stages of the question. On examining the answers given by control group students, it was found that the proportion fall from 33.90% to 24%. To the statement “The direction of electron flow is from Ag electrode in the first container to Ag electrode in the second container in the external circuit” 83.93% of the experimental group students and 42.37% of the control group students gave correct answers. In the ECT post-test, 8.93% of the experimental group students and 11.86% of the control group students were found to have alternative conceptions as “the potential of a battery is not dependent on the concentration of anode and cathode containers. Electricity power is needed in order for the battery to work, and the potential of the battery is negative according to Nernst equation”. And on examining the other distractors, it was found that 12.50% of the experimental group students and 6.78% of the control group students had alternative conceptions as “because there are no net reactions in concentration cells, reaction quotient cannot be calculated.”; and 5.36% of the experimental group students and 8.47% of the control group students had alternative conceptions as “cell potential in concentration cells is independent of the concentration of half-cell solutions”. Besides, the alternative “the direction of electron flow is independent of the concentration of solutions in cells” was chosen only by 16.95% of the control group students. The proportion of correct answers at the second stage was 73.21% in the experimental group and 49.15% in the control group.
In question 12, an attempt was made to determine whether or not students had alternative conceptions about galvanic cells. While 75% of the experimental group students answered the first two stages of the question correctly, 57% of them said that they were sure of their answers. As to the answers given by control group students, it was found that the proportion dropped from 28.81% to 25%. To the statement “the direction of spontaneous change is that of the forward reaction”, 96.64% of the experimental group students and 66.10% of the control group students responded correctly. In the ECT post-test, 1.79% of the experimental group students and 3.39 % of the control group students had alternative conceptions as “electrons enter the electrolyte from the cathode, they are carried by electrolyte and salt bridge, and then they complete the circuit and they emerge in the anode”. 1.79% of the experimental group students and 5.08% of the control group students based their answers to the statement “electrons can move in solutions just like ions do” and 3.57% of the experimental group students and 25.42% of the control group students based their answers to the statement “conductivity in solutions is ensured only through the movement of negatively charged ions”. Another distractor, one of the alternatives, “anode is always on the left” was chosen by 8.93% of the experimental group students and 15.25% of the control group students. The proportion of correct answers at the second stage of the question was found to be 78.57% in the experimental group and 44.07% in the control group.
Table 2. Alternative conceptions Found by Means of ECT Post-test in the Experimental and Control Groups, and Their Percentages
Student’s alternative conceptions
Question
Post-test Experimental group
Post-test Control group
%
%
Conductivity in solutions is ensured only through the movement of negatively charged ions
12
3.57
25.42
Electrons can move in solutions just like ions do.
6
1.79
5.08
Anode is always on the left.
12
8.93
15.25
The place of anode and cathode is determined by the physical placement of half-cells.
5
7.14
5.08
Only negatively charged ions form a flow of current in electrolyte and salt bridge.
12
1.79
6.78
Electrons flow in aqueous solutions without assistance of ions.
12
1.79
11.86
Electrons enter the electrolyte from the cathode, they are carried by electrolyte and salt bridge, and then they complete the circuit and they emerge in the anode.
10
1.79
3.39
Anode is positively charged, because it releases electrons; cathode is negatively charged because it attracts electrons.
12
.00
6.78
Electrons’ direction of flow is independent of the concentration of solutions in concentration cells.
11
.00
16.95
In a voltaic battery cell, electrons flow from cathode to anode through a conducting wire.
7
3.57
5.08
Anions in the electrolytes attract electrons and transfer them into cathodes.
10
1.79
13.56
Since half-cell potentials are the values which are obtained by measuring, they are precise and are used in calculating half-cells
8
7.14
11.86
Changes in the charges of OH-, BiO3- and Cr(OH)4- ions determine the number of attracted and released electrons.
4
.00
8.47
Oxidation is the addition of oxygen whereas reduction is moving the oxygen away.
4
.00
11.86
Reduction half reaction for the reaction 2OH- + 3BiO3- + 2Cr(OH)4- → 3BiO2- + 2CrO42- + 5H2O is 2Cr(OH)4- + 1e- →2CrO42-
4
7.14
13.56
It was found that standard electrode potential for H2(1atm)/H+(1M) was zero in consequence of experimental measurements.
8
26.79
25.42
Students’ responses to ECT post-test were analysed for the second sub-problem of the research. In consequence, it was found that the students in the experimental and in the control group in this research also had alternative conceptions similar to the ones found earlier in the literature. Some of the alternative conceptions found in this study are as in the following:
- Changes in the charge of polyatomic types in an equation can be used in describing redox equations (Garnett, & Treagust, 1992a; 1992b).
- Oxidation is the addition of oxygen whereas reduction is moving the oxygen away (Garnett, & Treagust, 1992a;1992b; Sanger & Greenbowe, 1997a).
- The standard electrode potential for H2(1atm)/H+(1M) was zero was found in consequence of experimental measurements (Canpolat et al., 2004; Garnett & Treagust, 1992a; 1992b; Sanger & Greenbowe, 1997a; 1999).
- Inert electrodes can be oxidized or reduced (Sanger & Greenbowe, 1997a).
- Half-cell potentials are the values which are obtained by measuring, and they are precise and are used in calculating half-cells (Sanger & Greenbowe, 1997a).
- In a voltaic battery cell, electrons flow from cathode to anode through a conducting wire (Sanger & Greenbowe, 1997b; Taşdelen, 2011).
- Electrons are carried by electrolytes (Canpolat et al., 2004; Garnett & Treagust, 1992a; 1992b; Sanger & Greenbowe, 1997b; 1999).
- Electrons are carried by electrolyte and salt bridge (Acar & Tarhan, 2007; Garnett & Treagust, 1992a; 1992b; Taşdelen, 2011; Yang et al., 2003).
- The place of anode and cathode is determined by the physical placement of half-cells (Acar & Tarhan, 2007; Sanger & Greenbowe, 1997a; Taşdelen, 2011).
- Anode is positively charged, because it releases electrons; cathode is negatively charged because it attracts electrons (Acar & Tarhan, 2007; Garnett & Treagust, 1992a; 1992b; Taşdelen, 2011; Yang et al., 2003).
- Only negatively charged ions form a flow of current in electrolyte and salt bridge (Acar & Tarhan, 2007; Sanger & Greenbowe, 1997b; Yang et al., 2003).
- Electrons’ direction of flow is independent of the concentration of solutions in concentration cells (Sanger & Greenbowe, 1997a).
- In concentration cells, battery potential is independent of the concentration of solutions in half-cells (Canpolat et al., 2004; Sanger & Greenbowe, 1997b; Taşdelen, 2011).