Asia-Pacific Forum
on Science Learning and Teaching, Volume 12, Issue 2, Article 8 (Dec., 2011) |
Results of Descriptive Statistics
Results of descriptive statistics were interpreted under categories, that is; the percentage of correct responses on the CCI ranged from 4.5 % to 75.0 % and this range was divided into three intervals as the common procedure in item analyses. There were seven items in the 50-75 % interval, eight items in the 30-50 % interval and the remaining six items correspond to the interval of 4-30 % (Table II).
Table II: Items with respect to the intervals they located
Intervals (%)
50-75
30-50
4-30
Items
2, 4, 8, 10, 12, 13, 15
3, 6, 7, 9, 11, 14, 16, 18
1, 5, 17, 19, 20, 21
Both the 50-75 % and 4-30 % intervals were analyzed deeply in terms of students’ percentages of correct responses and their possible alternative conceptions in accordance with the purpose of this study. Table III indicates the percentages of responses on choices of items that fall into 50-75 interval.
Table III: The percentages of student responses on each alternative choices and standard deviations within the 50-75 interval
Test Items
A (%)
B (%)
C (%)
D (%)
E (%)
Standard deviation
2
2.3
23,9
3.4
69.3*
1.1
0.94
4
2.3
4.5
23.9
68.2*
1.1
0.70
8
20.5
13.6
10.2
50.0*
5.7
1.30
10
15.9
29.5
54.5*
-
-
0.74
12
5.7
12.5
75.0*
4.5
2.3
0.68
13
10.2
75.0*
8.0
2.3
4.5
0.81
15
59.1*
17.0
19.3
4.5
-
0.93
* indicates the correct choice of that item; - indicates items that do not have such a responseAs can be seen from Table III, the highest percentage (75.0 %) was shared by items 12 and 13, which were paired in nature (Figure I).
Figure I: Sample items from the CCI
Item 12. 1 gram of solid iodine is put into a sealed tube and the tube is closed after vacuumed. The total weight of solid iodine and the tube is 27 gram. What will be the total weight after the tube is heated and all of the iodine vaporized?
- Less than 26 gram
- 26 gram
- 27 gram
- 28 gram
- More than 28 gram
Item 13. What is the reason for your answer?
- A gas weighs less than a solid.
- Mass is conserved.
- A gas is less dense than a solid.
- Gases rise.
Item 12 investigates whether or not students could comprehend the conservation of mass of solid iodine as it changes to iodine vapor by heating. Seventy-five percent of students marked “The mass would be the same”, but the remaining thought as “The mass would be less or more”. The reason of answer given to the 12th item was asked in 13th item and same percentage (75 %) of students marked “Mass is conserved” as the result. Except correct response, the percentage of the most common response was approximately 10 %, which states that “A gas weighs less than a solid”. This alternative conception is analogous to results of related studies reported in related literature (Stavy 1988, 1990; Mas et al. 1987; Lee et al. 1993).
Item 2 explores the contents of bubbles in boiling water and about 70 % of the students stated the content as “Water vapor”. Besides correct answer, the most common answer was “The oxygen and hydrogen gases” (24 %), which is consistent with the related literature (Costu et al., 2007). About 68 % of students gave correct answer (Mass of a solution equals to sum of the masses of solute and solvent) to the 4th item that asks about conservation of mass during dissolution. Among the remaining respondents, nearly 24 % of them indicated “A value between mass of solvent and total mass of solute and solvent” as the correct answer, which indicates that they have alternative conceptions about the law of “conservation of mass”. Item 15 asks about heat of water and alcohol having equal temperature and mass after being heated a while, and nearly 59 % of respondents replied correctly whereas approximately 20 % of them confused heat and temperature by selecting the common alternative conception “both of them receive same amount of heat”. Many research studies also indicated the confusion of heat and temperature concepts by students (Erickson, 1979; Kesidou & Duit, 1993; Harrison, Grayson & Treagust, 1999; Niaz, 2000; Yeo & Zadnik, 2001). Item 10 seeks the change in water level as ice melts in a mixture of water and ice and about 55 % of pupils stated the correct answer “It stays the same” and the remaining answered as “Decreases”, “Increases” (almost 30 % and 16 %, respectively).
Interestingly, the item 8 had higher percentage of correct response (50 %) than item 7 (nearly 47 %), which were paired questions and the former explores the reason of question 7 (true or false type question) in case of whether or not matter is destroyed as a match burns. The percentages of other choices for item 8 suggests that some of the respondents are not able to comprehend chemical change but they recall changes during chemical reactions such as “The atoms are not destroyed, they are only rearranged” (Figure II).
Figure II: Sample items from the CCI
Item 7. Specify the following sentence as True or False; “There is matter lost as match burns”
- True
- False
Item 8. What is the reason for your answer to the above question?
- This chemical reaction terminates matter
- Matter is consumed by fire
- Mass of ash is less then mass of match
- Atoms do not dissappear but re-arranged
- Weight of burned match is less then its initial mass
Up to here, the items within the 50-75 interval were introduced and now, it is the place to submit results related to the items that fall into 4-30 interval. Table IV presents the related items.
Table IV: The percentages of student responses on each alternative choices and standard deviations within the 4-30 interval
Test Items
A (%)
B (%)
C (%)
D (%)
E (%)
Standard deviation
1
33.0
10.2
10.2
14.8*
30.7
1.69
5
15.9
15.9
20.5
4.5*
43.2
1.55
17
50.0
17.0
20.5*
12.5
-
1.10
19
67.0
12.5
19.3*
-
-
0.80
20
44.3
13.6*
33.0
8.0
-
1.05
21
11.4
4.5
22.7*
48.9
11.4
1.12
* indicates the correct choice of that item; - indicates items that do not have such a response
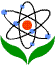
As can be seen from Table IV, the poorest percentage belongs to item 5 that investigates chemical formulas and equations.
Figure III: Sample item from the CCI
Item 5. The larger diagram at the top represents a mixture of S atoms and O2 molecules in a closed container. Which diagram (a-e) shows the result after the mixture reacts as completely as possible according to the equation 2S + 3O2 → 2SO3 ?
The correct answer was selected by 4.5 % of respondents, solely. Percentage of students who selected other choices (a, b, c, and e) is about 95 % which can be interpreted as students are unable to comprehend conservation of atoms in chemical reactions. Students who selected “a, b, and e” (75 %) do not know about conservation of kinds of atoms during chemical reactions, and students who marked “c” as the answer (almost 21 %) are not aware of conservation of number of atoms during chemical reactions. Being unable to conserve kinds of atoms during chemical reactions may be the result of not knowing the difference between the meaning of coefficient (that is “2” in the item, 2SO3) and subscript (that is “3” in the item, 2SO3). This result is in accordance with the study of Mulford and Robinson (2002).
Actually, the result for the item 5 is consistent with the item 1, which explores also the concepts of conservation of mass, molecules, and atoms during chemical reactions. Only 15 % of respondents comprehend the conservation of both the number of atoms and the total mass in chemical reactions. Students have common alternative conceptions such as “Only total mass is conserved in chemical reactions”, and “The total number of molecules is also conserved in chemical reactions”, which indicates confusion between molecules and atoms. Combining results of items 5 and 1 indicates that students have trouble especially with sub-microscopic and symbolic representations of chemistry concepts.
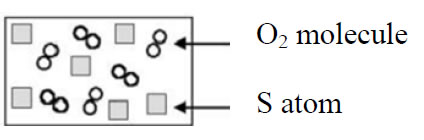
Items 19 and 20 are also paired questions, which asks about concentration characteristics of a saturated solution as water evaporates.
Figure IV: Sample items from the CCI
Item 19. Salt is added to water and the mixture is stirred until no more salt dissolves. The salt that does not dissolve is allowed to settle out. What happens to the concentration of salt in solution if water evaporates until the volume of the solution is half the original volume? (Assume temperature remains constant).
The concentration
- Increases
- Decreases
- Stays the same
Item 20. What is the reason for your answer?
- There is the same amount of salt in less water.
- More solid salt forms.
- Salt does not evaporate and is left in solution.
- There is less water.
Among students, only 19 % of them responded as “The concentration stays constant”, and only 13 % of them thought the reason of staying constant as “More solid salt forms”. Most of the students (67 %) marked “The concentration increases” as a result of “There is the same amount of salt in less water” (44 %), “Salt does not evaporate and is left in solution” (33 %), and “There is less water” (8 %). Respondents’ concepts related to the concentration of saturated solution after evaporation of water are poor whereas their states of reason are poorer which conveys the message that students can use chemical concepts without comprehending the logic behind those concepts. In other words, they do not learn meaningfully but rather recall some facts.
Item 17 investigates mass of a rusting iron nail and only, 21 % of students answered as “The rust would weigh more”. Half of the students responded as “The rust would weigh less”, 17 % believed that “The weight of rust would be the same”, and nearly 12 % thought as “It is impossible to predict”. An interesting result, when students were asked in order to explain reasons of changes related to mass of rust (Item 18), they gave higher correct percentages (almost 32 %).
Item 21 is the last question to be analyzed at the 4-30 interval which asks about the properties of a single sulfur atom by giving necessary properties of macroscopic sample of sulfur. Solely, 23 % of students responded as “It forms sulfur dioxide by combining with oxygen”. Most of the students (49 %) believed that “It has same properties with macroscopic sample of sulfur”. Attribution of macroscopic properties to microscopic entities (atoms in this case) are also reported as alternative conceptions of students in the related literature (Abraham et al., 1994; Griffiths & Preston, 1992; Novick & Nussbaum, 1981).
Results of Inferential Statistics
Two-Way ANOVA results indicated that there is no statistically significant effect of gender on pupils’ comprehension of chemistry concepts as F(1, 77)=0.028, p>0.05, as well as, there is no statistically significant effect of previous semester chemistry course grades on pupils’ comprehension of chemistry concepts as F(3, 77)=2.609, p>0.05. ANOVA results also showed that there is no interaction between gender and previous semester chemistry course grades as F(3, 77)=0.188, p>0.05.